Research
Neural circuit remodeling during development
Neural circuits are immature at the time of birth. Postnatally, an excess of synapses is produced, and redundant synapse is eliminated thereafter to form sophisticated neural circuits. We use rodent somatosensory thalamus as a model system to investigate the maturation process of the synapse during the development. To understand the functional aspects, we investigate both pre- and postsynaptic side. We are also interested in how growing environment or sex differences affect the neural circuit maturation process and their functions. We use genetics, optogenetics, immunohistology, in vivo and in vitro electrophysiology, and behavior analysis to address those questions.
Neural circuitry and molecular mechanisms underlying peripheral nerve injury-induced synaptic remodeling
Peripheral nerve injury or limb amputation frequently develops neuropathic pain such as phantom limb pain. The prevailing theory for development of phantom limb pain has been somatotopic reorganization of cortical and thalamic circuits induced by maladaptive plasticity. In this theory, neurons in the missing limb area become activated by invading ectopic inputs relaying somatosensory information from other body parts, which function as an error signal due to somatotopic mismatching. Consistent with this theory, using mice receiving peripheral nerve injury by whisker deafferentation or whisker nerve ligation, we have been reported functional relationships between somatotopic reorganization in the thalamus and ectopic mechanical hypersensitivity. We aim to identify mechanisms of peripheral nerve injury-induced synaptic remodeling across multiple levels of molecular, cellular, and circuit organization.
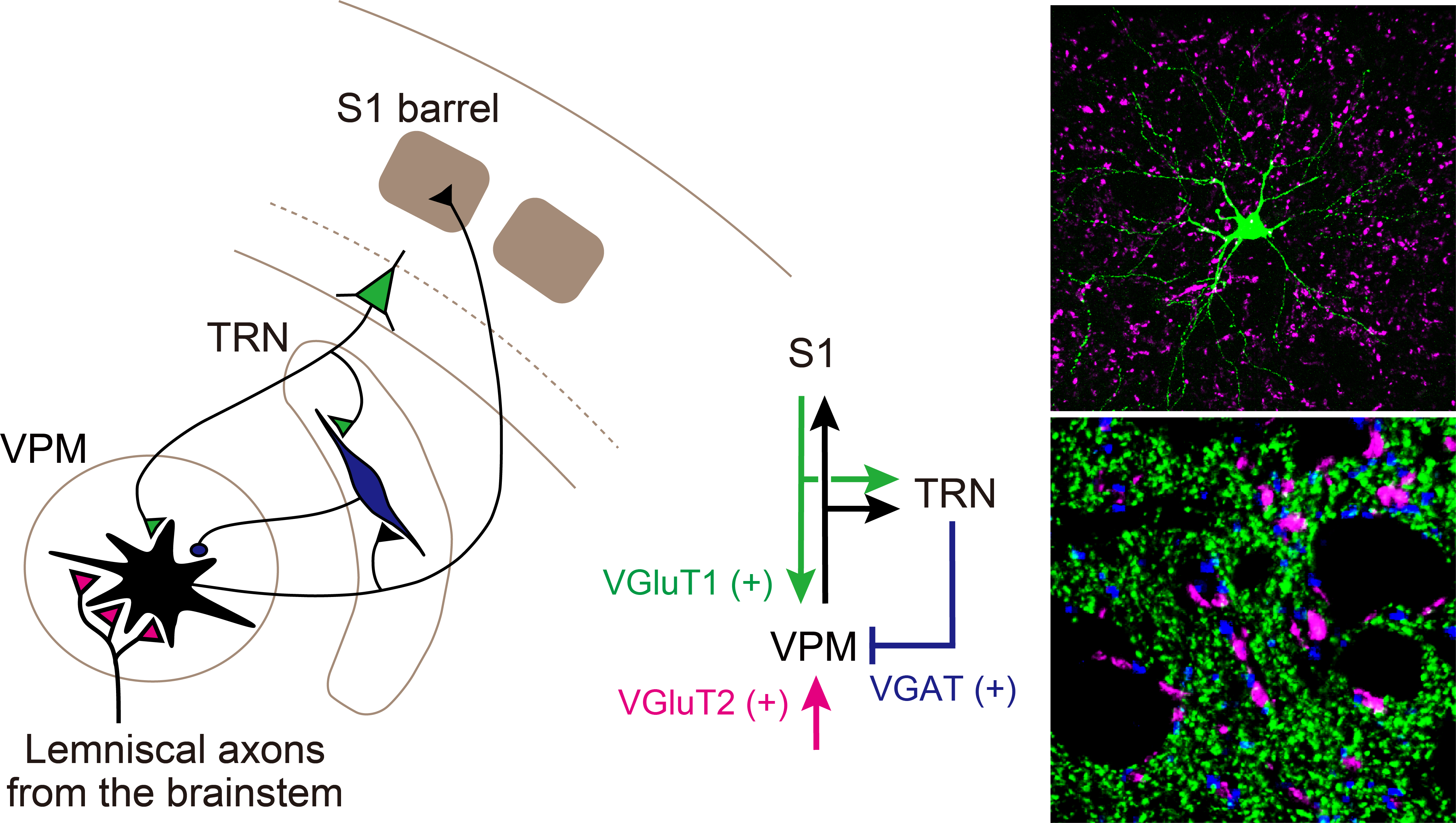
Figure: Circuit in the primary somatosensory thalamic nuclei
Neural Circuit of Central Pain
Neural mechanisms giving rise to pain are complex. Our lab studies the roles of the thalamocortical circuits in the neural transformation by which pain signals from the skin make painful subjective experiences. We discovered a specific area in the primary somatosensory cortex (dysgranular area) that preferentially encodes pain over tactile information. Blockade of signal transmission from the thalamus to the dysgranular area exerts an analgesic effect. These findings suggest that abnormally augmented thalamocortical transmission in this cortical area may account for "central pain“ . To understand the mechanisms of central pain and establish its cures, we deploy multiple technical approaches (e.g., mouse genetics, optogenetics, extracellular recording using high-density probes, behavioral analyses) to address the following questions: (1) How are pain signals transmitted to and gated at the thalamus? (2) Does the pain-relay thalamus integrate pain and other somatosensory signals such as touch and proprioception? (3) Do changes in the somatosensory thalamocortical pathway account for central pain in animal models?
Improvement of peripheral nerve regeneration using stem cells
Autologous nerve transfer has been reliable surgical treatment to achieve acceptable functional recovery, for example from facial nerve paralysis, after traumatic peripheral nerve injury. To overcome atrophy and degeneration of denervated muscles or organs due to a slow rate of natural nerve regeneration, rapid regeneration of functionally relevant peripheral nerves is required to reconstruct nerve gaps with autologous nerve grafts in clinical practice. We are investigating how stem cells improve peripheral nerve regeneration and functional recovery using rat models in collaboration with Department of Plastic and Reconstructive Surgery.